

The analytical development/QC center of Alphamab has the capability to cover full range of analysis and characterization of biologics. More than 100 state of art instrument were acquired over past 3 years, including HPLC, UPLC, DSC, Biacore, Optim II, cIEF, icIEF, TOC, particle analyzer, FTIR, fluo-spec,confocal, FACS, cell sorter, Q-TOF, stability chamber.
With well trained stuff, the AD/QC center has been developed and validated dozens of methods to support IND filing of several candidates, novel, biobetters and biosimilars.
1. Structure characterization
Primary structure: LC/MS/MS, peptide mapping, CE-SDS, N-Terminal Sequencing
Secondary Structure: CD, DSC, FTIR, DFS
PTM: Glycosyaltion and other PTM
2. QC and Release assay
Purity and charge profiling: SEC, cIEF, IEX,CE
Impurity and contaminant: HCP, residue DNA, deamidation, oxidation, glycation, aggregation, degradation
Others: pH, osmolarity, color,turbity,Viscosity
3. Formulation, stress, forced degradation and Stability
4. Biological activity
PPI based on ELISA and SPR
Cell based activity
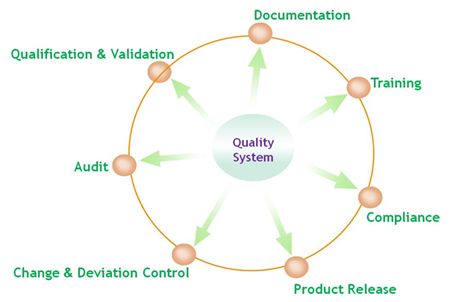 Quality system in Alpahamb roots in strict compliance to cGMP requirement of cFDA, FDA and EMA. The system covers all aspects from early stage research to product release, including R&D, manufacturing, quality control, risk mitigation, deviation management and quality assurance. The quality system in Alphamab , focusing on personnel training, is constantly evolving to meet the dynamic landscape of complicate regulatory environment.
Quality system in Alpahamb roots in strict compliance to cGMP requirement of cFDA, FDA and EMA. The system covers all aspects from early stage research to product release, including R&D, manufacturing, quality control, risk mitigation, deviation management and quality assurance. The quality system in Alphamab , focusing on personnel training, is constantly evolving to meet the dynamic landscape of complicate regulatory environment.