
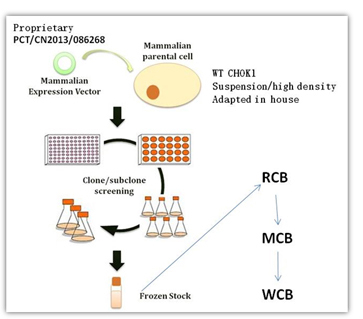
 Stable Clone Generation
Stable Clone Generation
Our high productive stable cell clones for biologics were derived from wide type CHOK1, preadapted to high density growth in serum free medium. CHOK1 cell are transfected with our patented expression vector and proprietary selection strategy. Eliminating the tedious gene amplification, we are able to get a stable subclone within 3 months. To be noted, the whole process is serum free. While speed and efficiency are highly regarded in Alphamab, we do slow down sometimes looking at critical attributes at first round of clone selection. This strategy has turned out to be critical for development of biosimilars.
Since 2008, Alphamab has successfully generated over 60 production clones, including mAbs, enzymes, glycol-hormones, cytokines, clotting factors, enzymes. For mAbs, we set high bar for clone selection, a 7 day batch culture with yield close to 0.7g/L prior to optimization. For particular projects, we also developed human 293 cell as the parental cell to ensure authentic human like PTM.
Process Development
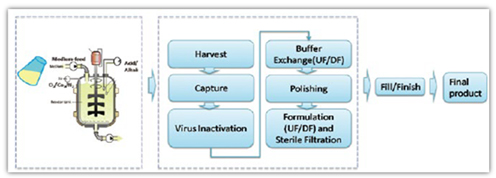
The process development capability is where Alphamab stands out. The development platform combine efficiently the cell culture, protein purification, virus removal/inactivation and cutting edge analytical capability. The DOE approaches have been applied during process development and optimization to improve the efficiency, reproducibility and scalability. QbD principle was adopted at early stage development focusing on PQA and CQA. The effort is supported systemically by PAT. The process development platform has track records for diversified products, e.g.,mAbs, clotting factors, fusion proteins and scaled up successfully to 1000L.

The cell culture PD team in Alphamab focuses on fed-batch process, with eye on scale up feasibility and PQA. The development course covers screening best basal media, media + feed and finally trace additives. The combination mostly identified in shake flask, further developed in parallel bioreactors(1L and 3L) with operation space defined by DOE experiments. Finally, those process parameters are confirmed in different size of bioreactors from 3L to 200L. The PD team has accumulated tremendous experience around this. It is routine for us to get titer over 2g/L for mAb expression, with several reach 8-10g/L over 13-16 day fed batch process.
 The DSP process team in Alphamab has broad experience on different class
of biologics, different type of antibodies, antibody fragments, nanobody,
fusion proteins, cytokines/hormones and difficult proteins like FVIII FVIIa and
many others . Supported by our deep understanding of biologics and
sophisticated analytical platform, our DSP development deemed to be highly
efficient and scaled up seamlessly.
The DSP process team in Alphamab has broad experience on different class
of biologics, different type of antibodies, antibody fragments, nanobody,
fusion proteins, cytokines/hormones and difficult proteins like FVIII FVIIa and
many others . Supported by our deep understanding of biologics and
sophisticated analytical platform, our DSP development deemed to be highly
efficient and scaled up seamlessly.  During process development, we put into consideration the quality of
products, efficiency, scalability and cost. As an example, we have successfully
developed non-protein A based capture scheme. Comparing with mainstream protein
A based purification, the cost of new process is only 1/10 with higher
efficiency.
During process development, we put into consideration the quality of
products, efficiency, scalability and cost. As an example, we have successfully
developed non-protein A based capture scheme. Comparing with mainstream protein
A based purification, the cost of new process is only 1/10 with higher
efficiency.
DSP Process validation complies strictly the QbD for scale up and scale down. The validation is a continuous process from discovery phase to commercial launch.